Easy Explain the Diffence Betweemisopes and the Same the Elent
Main Difference – Isotopes vs Isobars
An atom of a chemical element is always composed of a nucleus surrounded by an electron cloud. The nucleus is composed of protons and neutrons. The number of protons present in an atom of a particular element is always the same. This means the number of protons is a unique property of a chemical element. However, the number of neutrons present in the nucleus can vary in atoms of the same element. These different forms are called isotopes. The atomic mass of a particular element is the sum of protons and neutrons in the nucleus of that element. Sometimes, the atomic masses of different elements can be the same although their atomic numbers are different from each other. These different forms are called isobars. The main difference between isotopes and isobars is that the atomic masses of isotopes are different from each other whereas the atomic masses of isobars are similar to each other.
Key Areas Covered
1. What are Isotopes
– Definition, Properties, Examples
2. What are Isobars
– Definition, Properties, Examples
3. What is the Difference Between Isotopes and Isobars
– Comparison of Key Differences
Key Terms: Atomic Mass, Atomic number, Isobars, Isotopes, Nucleon, Neutron, Proton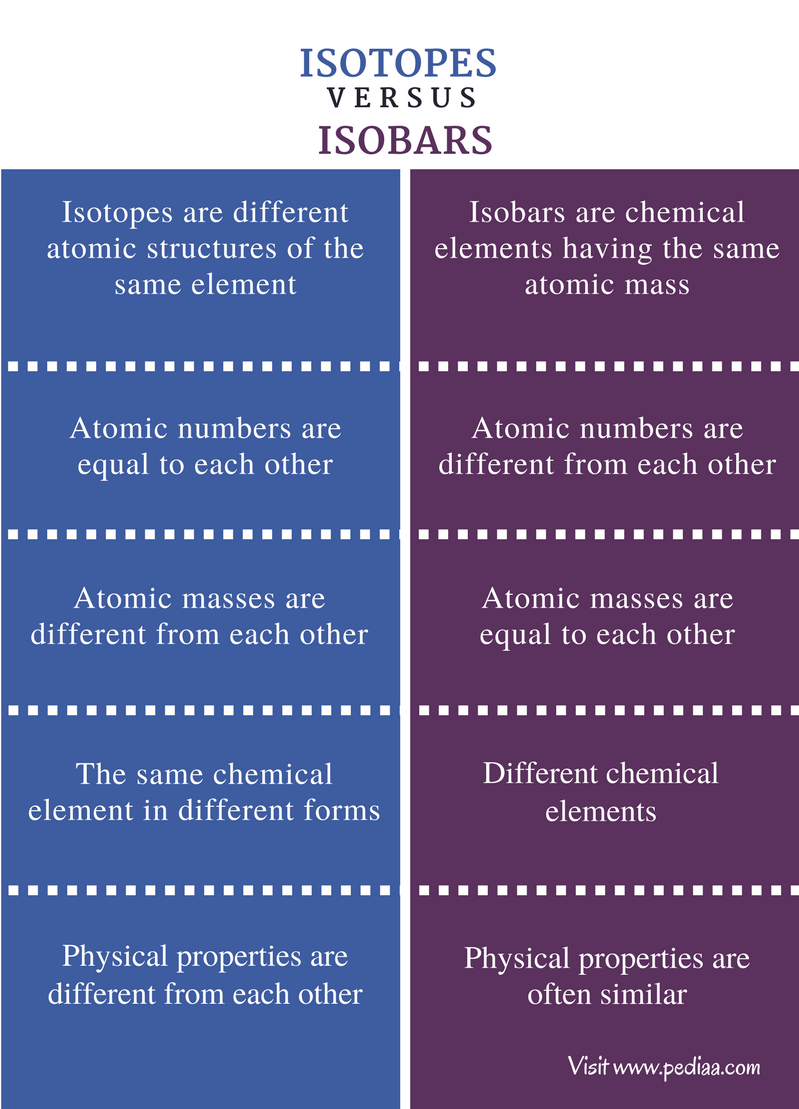
What are Isotopes
Isotopes are different atomic structures of the same element. This means isotopes have the same atomic number but different atomic masses. This is because the atomic number is a unique property for a chemical element due to the presence of the same number of protons in the nucleus. But the atomic masses of isotopes may differ from each other since the atomic mass is dependent on the number of neutrons present in the nucleus and isotopes have different numbers of neutrons.
Isotopes show similarities in their chemical properties since the chemical properties of an element is mostly dependent on the electrons present in atoms. Isotopes of an element have the same number of electrons and the same electron configuration.
The physical properties of isotopes may differ from each other. Generally, the physical properties are dependent on the atomic mass and isotopes have different atomic masses. Some examples of isotopes include the isotopes of Hydrogen, Helium, Carbon, Lithium, etc.
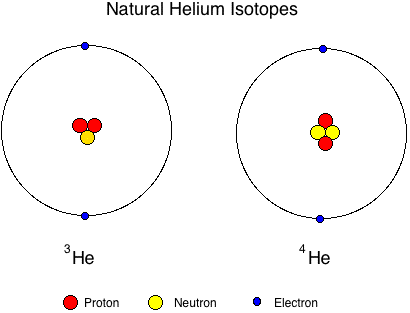
Figure 01: Naturally occurring isotopes of Helium
The above image shows the atomic structures of naturally occurring Isotopes of Helium. Here, one Helium isotope is composed of 1 neutrons whereas the other one is composed of 2 neutrons. But both of them have the same atomic number, 2. The atomic masses are different.
What are Isobars
Isobars are chemical elements having the same atomic mass. The atomic mass of a chemical element is given by the sum of the number of protons and neutrons. A proton or a neutron alone is called a nucleon. Therefore, the number of nucleons equals the atomic mass. Hence, isobars have the same number of nucleons. However, the numbers of protons and neutrons alone are always different from each other in isobars.
Isobars are composed of the same number of electrons. This indicates that isobars show same chemical properties. The physical properties are also the same because atomic masses are the same. Since the atomic numbers are different, isobars are different chemical elements.
Eg: A series of Isobars having the atomic mass of 64.
- Cobalt (Co)
- Nickel (Ni)
- Copper (Cu)
- Iron (Fe)
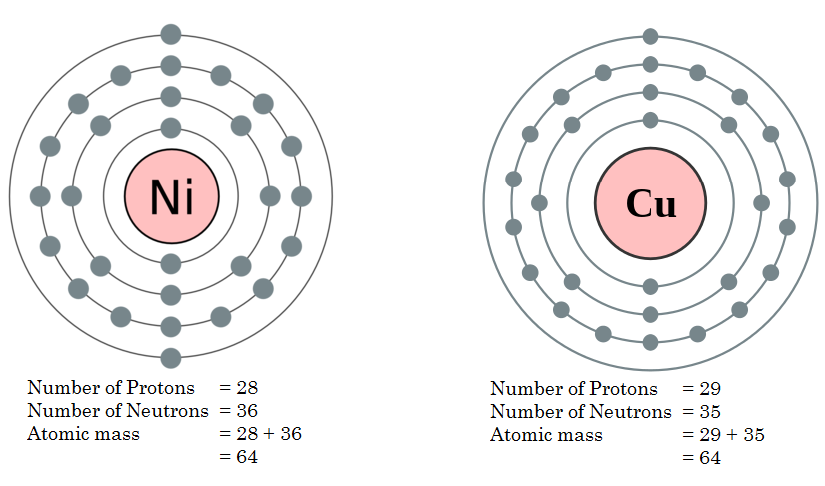
Figure 2: Atomic Masses of Copper and Nickel
Isobars having the atomic mass 3.
- Hydrogen (H)
- Helium (He)
Difference Between Isotopes and Isobars
Definition
Isotopes: Isotopes are different atomic structures of the same element.
Isobars: Isobars are chemical elements having the same atomic mass.
Atomic Number
Isotopes: The atomic numbers of isotopes are equal to each other.
Isobars: The atomic numbers of isobars are different from each other.
Atomic Mass
Isotopes: Atomic masses of isotopes are different from each other.
Isobars: Atomic masses of isobars are equal to each other.
Chemical Element
Isotopes: Isotopes are the same chemical element in different forms.
Isobars: Isobars are different chemical elements.
Physical Properties
Isotopes: The physical properties are different from each other in isotopes.
Isobars: Most of the physical properties are similar in isobars.
Conclusion
Isotopes and isobars are examples for the relationships between chemical elements. Isotopes refer to different forms of the same chemical elements. Isobars refer to the relationship between some different chemical elements sharing the same property. Therefore, it is very important to understand the difference between isotopes and isobars.
References:
1. "What is an Isobar?" Study.com. Study.com, n.d. Web. Available here. 20 July 2017.
2. Helmenstine, Ph.D. Anne Marie. "What Is an Isotope? Definition and Examples." ThoughtCo. N.p., n.d. Web. Available here. 20 July 2017.
Image Courtesy:
1." Helium-3 and Helium-4″ By Uwe W. – Own work (Public Domain) via Commons Wikimedia
2. "Electron shell 029 Copper" (CC BY-SA 2.0 uk) via Commons Wikimedia
3. "Electron shell 028 nickel" (CC BY-SA 2.0 uk) via Commons Wikimedia

Source: https://pediaa.com/difference-between-isotopes-and-isobars/
0 Response to "Easy Explain the Diffence Betweemisopes and the Same the Elent"
Post a Comment